OEP produces and markets a diverse range of products from generics to specialty pharma. Our portfolio focuses on complex modified release oral and cytotoxic injectables.
Our products are made in Taiwan, a Trade Agreement Act (TAA)-compliant country, allowing us to sell directly to the U.S. government.
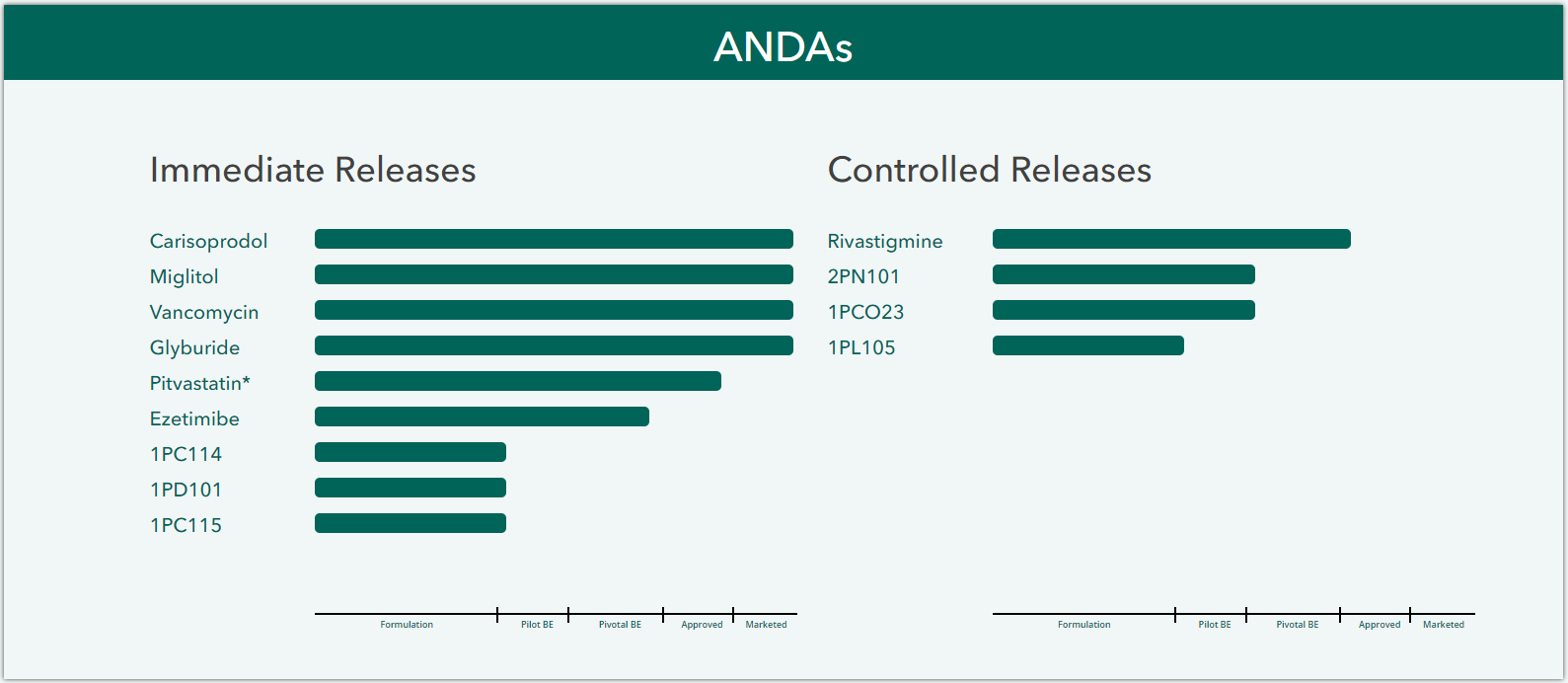
(2)s%20Progress%20bar.png)
© 2020 OEP Pharma. All right reserved.